The reaction of 44.1 g of Cr203 with 35.0 g of Al produced 25.6 g of Cr. What is the percent yield for this reaction?
2Al + Cr203 + Al203 + 2Cr
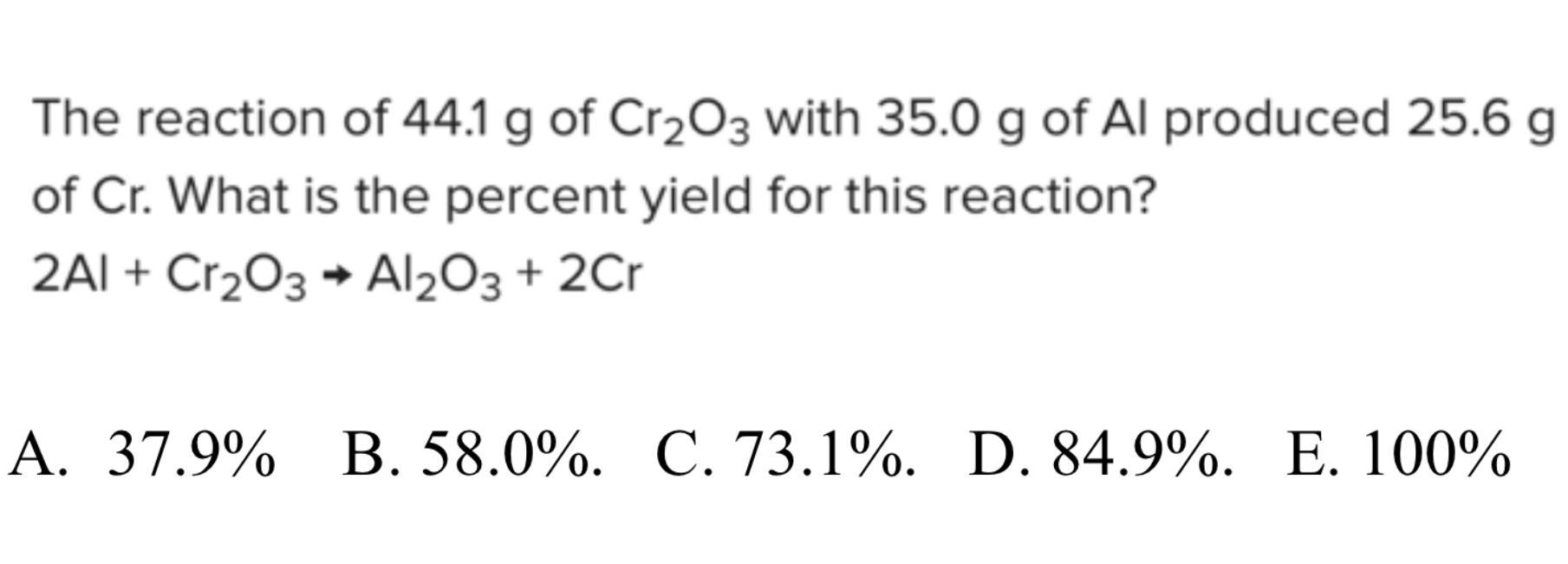
Answers
To determine the percent yield, we need to first calculate the theoretical yield of the reaction using stoichiometry, and then divide the actual yield by the theoretical yield and multiply by 100%. The percent yield of the reaction is approximately 84.9%.
What is percent yield?Percent yield is a measure of the efficiency of a chemical reaction, calculated by dividing the actual yield of a reaction by the theoretical yield and multiplying by 100%. It represents the percentage of the theoretical amount of product that was actually obtained in a reaction.
The balanced chemical equation is:
2Al + Cr₂O₃ → Al₂O₃ + 2Cr
The molar mass of Cr₂O₃ is 152 g/mol, the molar mass of Al is 27 g/mol, and the molar mass of Cr is 52 g/mol.
We need to determine which reactant is limiting, so we can calculate the theoretical yield based on the amount of limiting reactant. We can do this by calculating the number of moles of each reactant using their molar masses and dividing by their stoichiometric coefficients in the balanced equation:
moles of Cr₂O₃= 44.1 g / 152 g/mol = 0.29 mol
moles of Al = 35.0 g / 27 g/mol = 1.30 mol
From the balanced equation, we see that 1 mole of Cr2O3 reacts with 2 moles of Cr. Therefore, the theoretical yield of Cr is:
moles of Cr produced = 0.29 mol Cr₂O₃x (2 mol Cr / 1 mol Cr₂O₃) = 0.58 mol Cr
mass of Cr produced = 0.58 mol Cr x 52 g/mol = 30.16 g Cr
The percent yield is:
% yield = (actual yield / theoretical yield) x 100%
% yield = (25.6 g Cr / 30.16 g Cr) x 100% = 84.9%
Therefore, the percent yield of the reaction is approximately 84.9%.
To find out more about percent yield, visit:
https://brainly.com/question/17042787
#SPJ1
Related Questions
a good extraction solvent will have all the listed qualities except one. which quality listed is incorrect?
Answers
A good extraction solvent will have the following qualities: Low boiling point, High boiling point, High density, Low density, Solubility in water, Solubility in organic solvents, etc.The incorrect quality listed is high boiling; a good extraction solvent should instead have low selectivity.
Extraction is a technique used to separate a desired substance from a mixture. The method involves dissolving one or more compounds present in a sample into a solvent. Extraction can be used to separate a mixture into its individual components, extract a compound from a sample, or remove impurities from a product.The listed qualities of a good extraction solvent are as follows:
Low boiling point
High boiling point
High density
Low density
Solubility in water
Solubility in organic solvents
Ability to separate from the mixture
A good extraction solvent will have all the qualities listed above except one, which is "high boiling point." A good extraction solvent should have a low boiling point to allow easy separation from the mixture. It should also have high solubility in both water and organic solvents, enabling it to dissolve a wide range of compounds.A good extraction solvent should have high density, enabling it to form a clear layer when mixed with the sample. It should also have low density to enable the separation of the solvent and the extracted compound. Finally, a good extraction solvent should have the ability to separate from the mixture after extraction, which means it should not form an azeotrope with the compound to be extracted.
For more such questions on extraction solvent , Visit:
https://brainly.com/question/25418695
#SPJ11
The complete questions is :
A good extraction solvent will have all the listed qualities except one. which quality listed is incorrect?
Low boiling pointHigh boiling pointHigh densityLow densitySolubility in waterSolubility in organic solventsAbility to separate from the mixturefluorine gas and water vapor react to form hydrogen fluoride gas and oxygen. what volume of hydrogen fluoride would be produced by this reaction if of fluorine were consumed?
Answers
A volume of 2.28 liters of hydrogen fluoride would be produced by this reaction if 1 gram of fluorine was consumed.
The balanced chemical equation for the reaction:
F₂(g) + H₂O(g) → 2HF(g) + O₂(g)
From this equation, we see that 1 mole of fluorine reacts to form 2 moles of hydrogen fluoride.
The given mass of fluorine is not provided in the question. Let's suppose the mass of fluorine is 1 gram.
To convert 1 gram of fluorine to moles, we will use its molar mass. The molar mass of fluorine is 18.998 g/mol.
Hence,1 g F₂ × (1 mol F2/18.998 g F₂) = 0.0526 mol F₂
Since 1 mole of F2 reacts to form 2 moles of HF, the number of moles of HF produced will be:
0.0526 mol F₂ × (2 mol HF/1 mol F₂) = 0.1052 mol HF
We need to assume some values for pressure and temperature. Let's assume that the pressure is 1 atm and the temperature is 273 K.
We will also need to know the volume of water vapor involved in the reaction.
Let's suppose that the volume of water vapor is 1 L.
Using these assumptions, we can calculate the volume of hydrogen fluoride as follows:
PV = nRT
Where P = 1 atm, V is the volume of HF, n = 0.1052 mol, R = 0.0821 L atm/mol K, and T = 273 K.
Substituting these values, we get:
V = (nRT)/P = (0.1052 mol × 0.0821 L atm/mol K × 273 K)/1 atm = 2.28 L
Therefore, 2.28 liters of hydrogen fluoride would be produced by this reaction if 1 gram of fluorine was consumed.
Learn more about molar mass here:
https://brainly.com/question/837939
#SPJ11
does the hydrogen necessary in the electron transport chain come from the splitting of carbon dioxide molecules
Answers
The hydrogen necessary for this process is ultimately derived from the splitting of carbon dioxide molecules. Yes, the hydrogen necessary for the electron transport chain is derived from the splitting of carbon dioxide molecules in a process known as the Calvin Cycle, or the light-dependent reaction.
In this process, carbon dioxide, water, and light energy are used to create high-energy molecules, such as ATP and NADPH, which are then used in the electron transport chain. During the Calvin cycle, carbon dioxide is reduced by NADPH and ATP to produce a three-carbon molecule called glycerate 3-phosphate.
Hydrogen is removed from glycerate 3-phosphate to create a two-carbon compound known as glyceraldehyde 3-phosphate. This compound is then used to create other compounds, such as glucose, which can be used for energy.
Know more about electron transport chain here:
https://brainly.com/question/24372542
#SPJ11
You have been saving pennies in a jar, and you now have 125 pennies. You want to know the total mass of the pennies before you take them to the bank. If the average penny has a mass of 2.50 g, what is the total mass of the pennies?
Answers
Total mass = number of pennies x mass per penny
Given that you have 125 pennies, and the average penny has a mass of 2.50 g, we can plug in these values to get:
Total mass = 125 x 2.50 g
Total mass = 312.50 g
Therefore, the total mass of the pennies is 312.50 grams.
how many ml of 0.100 m nacl would be required to make a 0.0595 m solution of nacl when diluted to 150.0 ml with water?
Answers
89.25 mL of 0.100 M NaCl would be required.
Moles of NaCl in the final solution= (150.0 mL) (0.0595 M NaCl) = 8.925 mmol NaCl
We'll have to use the given 0.100 M NaCl and use its concentration to calculate the amount required to make 8.925 mmol NaCl.
The concentration of NaCl in moles per milliliter is as follows:
The concentration of NaCl in moles per mL = 0.100 M NaCl / 1000 mL/L = 0.0001 moles/mL NaCl
The volume of 0.100 M NaCl that contains 8.925 mmol NaCl is calculated as follows:
The volume of 0.100 M NaCl = (8.925 mmol NaCl) / (0.0001 mol/mL) = 89.25 mL
Therefore, 89.25 mL of 0.100 M NaCl is required to make 0.0595 M NaCl solution when diluted to 150.0 mL with water.
To learn more about solutions refer - https://brainly.com/question/29522675
#SPJ11
Given the solubility rules from the book, which of the following metal hydroxides should be soluble in water? LiOH CuOH AgOH. Cu(OH)2 TlOH. LiOH.
Answers
The metal hydroxide that should be soluble in water among LiOH, CuOH, AgOH, Cu(OH)₂, and TlOH is LiOH.
1. LiOH: Lithium hydroxide (LiOH) is an alkali metal hydroxide, and alkali metal hydroxides are generally soluble in water. So, LiOH is soluble.
2. CuOH: Copper(I) hydroxide (CuOH) is a transition metal hydroxide, which are typically insoluble. Therefore, CuOH is not soluble.
3. AgOH: Silver hydroxide (AgOH) is also a transition metal hydroxide and is insoluble in water.
4. Cu(OH)₂: Copper(II) hydroxide (Cu(OH)₂) is another transition metal hydroxide and is insoluble in water.
5. TlOH: Thallium hydroxide (TlOH) is also a transition metal hydroxide, and like most transition metal hydroxides, it is insoluble in water.
In conclusion, among the given metal hydroxides, LiOH is soluble in water.
To know more about metal hydroxide, refer here:
https://brainly.com/question/14407261#
#SPJ11
when hydrochloric acid reacts with barium hydroxide, barium chloride and water are produced. the balanced equation for this reaction is:
Answers
When hydrochloric acid reacts with barium hydroxide, barium chloride and water are produced. The balanced equation for this reaction is: `HCl + Ba(OH)₂ ⟶ BaCl₂ + H₂O `.
This is a neutralization reaction in which an acid and a base react to form salt and water. The acid in this case is hydrochloric acid and the base is barium hydroxide.
Hydrochloric acid (HCl) is a strong acid, which means that it ionizes completely in water. This means that it dissociates into hydrogen ions (H+) and chloride ions (Cl-) when dissolved in water.
The balanced equation for the ionization of hydrochloric acid is: `HCl + H₂O ⟶ H₃O⁺ + Cl⁻ `Barium hydroxide (Ba(OH)₂) is a strong base, which means that it ionizes completely in water.
This means that it dissociates into barium ions (Ba2+) and hydroxide ions (OH-) when dissolved in water. The balanced equation for the ionization of barium hydroxide is:` Ba(OH)₂ ⟶ Ba²⁺ + 2OH⁻`
When hydrochloric acid and barium hydroxide are mixed together, they react to form barium chloride (BaCl₂) and water (H₂O). The balanced equation for this reaction is:` HCl + Ba(OH)₂ ⟶ BaCl₂ + H₂O`
In this reaction, the hydrogen ion (H+) from the hydrochloric acid combines with the hydroxide ion (OH-) from the barium hydroxide to form water.
The barium ion (Ba2+) from the barium hydroxide combines with the chloride ion (Cl-) from the hydrochloric acid to form barium chloride.
To know more about Hydrochloric acid refer here:
https://brainly.com/question/14006357#
#SPJ11
Please answer both
The heat of vaporization for water is 2260 J/g. How much heat in J would be needed to evaporate 8.66g of water?
An unknown salt was dissolved to make a total 1.25g of solution. The temperature of the water decreased from 25.1C to 20.4C when 8mol were dissolved. What is the heat of solution in J/mol?
Answers
(a) We would need 19595.6 J of heat to evaporate 8.66 g of water.
(b) The heat of solution is -3.1 J/mol.
What is the heat needed to evaporate the water?To evaporate 8.66 g of water, we need to use the heat of vaporization for water, which is 2260 J/g.
Therefore, the total amount of heat required to evaporate 8.66 g of water is:
2260 J/g x 8.66 g = 19595.6 J
Therefore,
To find the heat of solution in J/mol, we need to use the formula:
ΔH_solution = -q_solution / n
where;
ΔH_solution is the heat of solution, q_solution is the heat released or absorbed during the solution process, n is the number of moles of solute dissolved.First, we need to calculate the heat released or absorbed during the solution process, which can be found using the formula:
q_solution = m_solution x C_solution x ΔT
We know that 8 mol of the unknown salt were dissolved in 1.25 g of solution, so the mass of the solute is:
m_solute = n x M
We also know that the temperature of the solution decreased from 25.1 ⁰C to 20.4 ⁰C, so ΔT = 4.7 K.
The specific heat capacity of water is 4.184 J/g·K, so we can assume that the specific heat capacity of the solution is also 4.184 J/g·K.
Therefore, the heat released or absorbed during the solution process is:
q_solution = 1.25 g x 4.184 J/g·K x 4.7 K = 24.8 J
Now we can use this value to calculate the heat of solution:
ΔH_solution = -q_solution / n
= -24.8 J / 8 mol
= -3.1 J/mol
Therefore, Note that the negative sign indicates that the solution process is exothermic, i.e., heat is released during the process.
Learn more about heat of solution here: https://brainly.com/question/29055794
#SPJ1
if a student decomposes 58.498 grams of aluminum carbonate and she collects the gas and measures 27.68 grams of carbon dioxide, determine the percent yield for this reaction. al2(co3)3 ----> al2o3 3co2
Answers
Answer : The percent yield for this reaction " al2(co3)3 ----> al2o3 3co2 " is 84.19%.
When a student decomposes 58.498 grams of aluminum carbonate, and collects gas and measures 27.68 grams of carbon dioxide, the percent yield for this reaction is calculated as follows; First, we find the theoretical yield of CO2 generated from the given Al2(CO3)3 based on stoichiometric calculations as given below; 2Al2(CO3)3 → 4Al + 6CO2 + 3O2Now, the molecular mass of Al2(CO3)3 is calculated as follows: 2(27) + 3(12 + 16x3) = 2(27) + 3(60) = 54 + 180 = 234 g/mol
Thus, the theoretical yield of CO2 generated from 58.498 g of Al2(CO3)3 is given by; moles of Al2(CO3)3 = 58.498 g / 234 g/mol = 0.2496 molTherefore, the moles of CO2 generated = 6 x 0.2496/ 2 = 0.7488 mol
The theoretical yield of CO2 generated from 58.498 g of Al2(CO3)3 is 32.91 g. Accordingly, the percent yield is given by; Percent yield = actual yield / theoretical yield x 100%, Where actual yield = 27.68 g and theoretical yield = 32.91 g. Therefore, Percent yield = 27.68 g / 32.91 g x 100% = 84.19%
Answer : The percent yield for this reaction is 84.19%.
Know more about aluminum carbonate here:
https://brainly.com/question/21544759
#SPJ11
From Portugal to Senegal use the currenct
Answers
1- From Portugal to Senegal, use the Canary Current
2- The current from #1 is a wind driven boundary surface current
What are different currents to sail around world?The ocean is divided into five major gyres (circulating currents). The North Pacific, South Pacific, North Atlantic, South Atlantic, and Indian Ocean gyres are the most well-known. The ocean's currents are made up of the water that circulates through each gyre.
3- From Senegal to Venezuela, use the S. Equatorial Current
4- From Venezuela, stay close to the coast and sail south with the Brazil Current to reach Argentina.
5- From Argentina, go west around Cape Horn against the Antarctic Circumpolar Current, and sail north with the Peru Current to reach Ecuador
6- Which current from #4 or 5 is an eastern boundary surface current? The Peru Current
7- Depart Ecuador on a westward course using the South Equatorial Current to reach Papua New Guinea
8- Sail through the Celebes and South China Seas to reach the Sri Lanka. Here you will pick up
to know more about water currents , visit ;
brainly.com/question/30162398
#SPJ1
if you start with 0.045 m of i2 at this temperature, how much will remain after 5.12 s assuming that the iodine atoms do not recombine to form i2 ? g
Answers
At 0.045 m of I2 and a given temperature, after 5.12 s of reaction, a certain amount of I2 will remain, the amount of I2 remaining, it is important to consider the rate of reaction of the: iodine atoms.
Assuming that the iodine atoms do not recombine to form I2, we can use the formula:
[tex]m(t) = m(0) x e^(-kt),[/tex]
where m(t) is the mass of I2 remaining after time t, m(0) is the initial mass of I2, k is the rate constant, and t is the time.
Therefore, the mass of I2 remaining after 5.12 s is [tex]0.045 m x e^(-k x 5.12 s).[/tex]
To solve for the rate constant k, we can use the equation
[tex]k = -ln(m(t)/m(0)) / t,[/tex]
where m(t) is the final mass of I2 and m(0) is the initial mass of I2.
Therefore, the rate constant for the reaction is [tex]-ln(m(5.12s)/m(0)) / 5.12s[/tex]. With this rate constant, the amount of I2 remaining after 5.12 s can be calculated by plugging it into the first equation, [tex]m(t) = m(0) x e^(-kt).[/tex]
To know more about iodine atoms refer here:
https://brainly.com/question/14077853#
#SPJ11
we decided to use 5.2 molar equivalents based on our past experience performing this type of reduction. what is the theoretical absolute minimum number of molar equivalents one could use in a sodium borohydride reduction of a ketone like camphor? (think about the structure of sodium borohydride). 2. calculate the % yield of the reaction, clearly showing your work.
Answers
The percent yield of the reaction is 80.6%. The actual yield is the amount of product actually obtained from the reaction, usually measured in grams or moles
What is Percentage Yield?
Percentage yield is a measure of the efficiency of a chemical reaction, and it represents the proportion of the actual yield of a product obtained from the reaction compared to the theoretical yield .
The stoichiometry of the sodium borohydride reduction of a ketone like camphor is as follows:
2 R₂C=O + NaBH₄ + 3 H₂O → 2 R₂CHOH + NaBO₂ + 4 H₂
From the balanced equation, we can see that 1 mole of sodium borohydride (NaBH₄) reacts with 2 moles of ketone (R₂C=O). Therefore, the theoretical absolute minimum number of molar equivalents of sodium borohydride required for the reduction is 1 equivalent per mole of ketone.
However, in practice, it is often necessary to use an excess of reducing agent to ensure complete reduction of the ketone. In the question, it is stated that the recommended amount is 5.2 molar equivalents based on past experience.
To calculate the percent yield of the reaction, we need to know the amount of product obtained and the theoretical yield of the product. The theoretical yield is the maximum amount of product that can be obtained based on the amount of limiting reagent used. In this case, the limiting reagent is the ketone, and the theoretical yield can be calculated as follows:
moles of ketone used = (mass of ketone used) / (molar mass of ketone)
theoretical yield of product = 2 x moles of ketone used
Once the actual yield of the product is obtained, the percent yield can be calculated using the formula:
For example, if we use 2 grams of camphor (molar mass 152.23 g/mol) and obtain 1.5 grams of the reduced product, the calculations would be:
moles of camphor used = 2 g / 152.23 g/mol = 0.0131 mol
theoretical yield of product = 2 x 0.0131 mol = 0.0262 mol
% yield = (1.5 g / (0.0262 mol x 88.15 g/mol)) x 100% = 80.6%
Therefore, the percent yield of the reaction is 80.6%.
Learn more about Percentage Yield from given link
https://brainly.com/question/2451706
#SPJ1
last time, you determined two important quantities for [fe(ncs)] 2 2 , what were these two quantities?
Answers
The two important quantities for [Fe(NCS)2]2- are its charge, which is -2, and its coordination number, which is 4.
What is Fe(NCS)22-?Fe(NCS)22- is a coordination complex with a central iron (II) cation that is surrounded by four water molecules and four bidentate NCS– ligands. It is a red-colored complex that is commonly used to evaluate ligand reactivity and to provide an understanding of the mechanisms of substitution reactions. It is formed by the reaction of FeSO4 with NaSCN in water. The formula for Fe(NCS)22- is Fe(H2O)4(NCS)22-.
The crystal field splitting energy is a measure of the energy difference between the lower and upper d-orbitals of an octahedral complex. This energy is determined by the electronic field that is created by the ligands surrounding the central metal ion. The crystal field splitting energy is an important quantity because it affects the optical and magnetic properties of a coordination complex.
Read more about the complex :
https://brainly.com/question/28007375
#SPJ11
devise a 6-step synthesis of a carboxylic acid from ethyne using the reagents provided. ethyne is a carbon carbon triple bond, bonded to two hydrogens. three reagents convert this to the main intermediate, an alkene with three bonds to hydrogen and one bond to a propyl group. three more reagents convert this to the product, which is a carboxylic acid bonded to a four carbon chain. reagent 1 is: reagent 2 is: reagent 3 is: reagent 4 is: reagent 5 is: reagent 6 is:
Answers
To synthesize a carboxylic acid from ethyne using the reagents provided, follow these steps: Hydroboration-oxidation, Tautomerization, Nucleophilic addition, Oxidation and Oxidative cleavage.
Hydroboration-oxidation- Reagent 1: Diborane (B2H6); Reagent 2: Hydrogen peroxide (H2O2) and sodium hydroxide (NaOH) Ethyne (C2H2) will undergo hydroboration-oxidation using diborane (B2H6) followed by treatment with hydrogen peroxide (H2O2) and sodium hydroxide (NaOH) to form an alkene (vinyl alcohol) with three bonds to hydrogen and one bond to a hydroxyl group.
Tautomerization- The vinyl alcohol formed in step 1 will undergo tautomerization (keto-enol equilibrium) to form an aldehyde with two carbons. Nucleophilic addition- Reagent 3: n-Propyl Grignard reagent (n-PrMgBr) Add the n-Propyl Grignard reagent (n-PrMgBr) to the aldehyde. This will result in a nucleophilic addition reaction, leading to the formation of a tertiary alcohol with a four-carbon chain.
Oxidation- Reagent 4: Chromic acid (H2CrO4), Oxidize the tertiary alcohol to a ketone using chromic acid (H2CrO4). This will form a ketone with a four-carbon chain. Oxidative cleavage- Reagent 5: Ozone (O3), Reagent 6: Zinc (Zn) and water (H2O), Perform an oxidative cleavage of the ketone using ozone (O3) followed by a reductive workup with zinc (Zn) and water (H2O). This will result in the formation of a carboxylic acid bonded to a four-carbon chain.
To know more about carboxylic acid, refer here:
https://brainly.com/question/29035899#
#SPJ11
there are three mechanistic steps of an aldol addition reaction: (1) deprotonation, (2) nucleophilic attack, (3) protonation.
Answers
The aldol reaction involves the reaction of an aldehyde or ketone with an enolate ion to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to form a double bond.
The aldol reaction is an important organic reaction in the formation of new carbon–carbon bonds. The reaction is named after the aldol reaction product, which contains both aldehyde and alcohol groups.
The aldol addition reaction has three mechanistic steps, which are deprotonation, nucleophilic attack, and protonation. These steps are explained below:
(1) Deprotonation: In the first step of the aldol reaction, the base removes a proton from the α-carbon of the carbonyl compound, which leads to the formation of the enolate ion.
The enolate ion is a resonance-stabilized anion that contains a negative charge on the oxygen atom and a double bond between the carbon and oxygen atoms.
(2) Nucleophilic attack: In the second step of the aldol reaction, the enolate ion acts as a nucleophile and attacks the carbonyl group of another molecule of the aldehyde or ketone.
This leads to the formation of a β-hydroxyaldehyde or β-hydroxyketone intermediate.
(3) Protonation: In the final step of the aldol reaction, the β-hydroxyaldehyde or β-hydroxyketone intermediate is protonated by the acid.
This leads to the formation of the aldol addition product, which contains a new carbon–carbon bond.
Thus, the aldol addition reaction involves three mechanistic steps, which are deprotonation, nucleophilic attack, and protonation.
These steps are essential for the formation of the aldol addition product, which contains a new carbon–carbon bond.
The aldol reaction is an important organic reaction that is widely used in the synthesis of natural products and pharmaceuticals.
to know more about aldehyde refer here:
https://brainly.com/question/30722723#
#SPJ11
was the weight of nylon a week later very different from the weight of nylon at the end of the lab period? provide a possible explanation.
Answers
The most significant commercially produced fibers include nylons.
Weight of nylon Nylon fibers are utilized in toothbrushes and tents, so chances are you've used them. Nylon may, however, be more than just fibers. Self-lubricating bearings and gears are also made with it. Automotive interior elements made of nylon-clay composites are utilized in vehicles.Nylon 6 and Nylon 6 are the two most significant varieties of nylon. Nearly all the features of these two nylons are the same. Both were developed in the late 1930s. First identified was nylon 6,6. Wallace Carothers, a DuPont employee, came up with the idea in the United States. 10 Paul Schlack, who was working for I.G. Farben at the time, soon after created Nylon 6 in Germany.For more information on nylon kindly visit to
https://brainly.com/question/10278626
#SPJ1
How many formula units are contained in 0. 67 grams of CaO?
Answers
There are approximately 7.15 x 10^21 formula units of CaO present in 0.67 grams of CaO.
Calculate the molar mass of CaO, which is the sum of the atomic masses of calcium and oxygen,
Molar mass of CaO = (1 x atomic mass of Ca) + (1 x atomic mass of O)
Molar mass of CaO = 56.08 g/mol
Convert the given mass of CaO to moles using the molar mass,
Moles of CaO = Mass of CaO / Molar mass of CaO
Moles of CaO = 0.0119 mol
Use Avogadro's number to convert moles of CaO to formula units,
Formula units of CaO = Moles of CaO x Avogadro's number
Formula units of CaO = 0.0119 mol x 6.022 x 10^23 formula units/mol
Formula units of CaO = 7.15 x 10^21 formula units
To know more about formula units, here
brainly.com/question/20704685
#SPJ4
is it possible to use the same colored central atom to make a model for all of these molecules? why?
Answers
Yes, it is possible to use the same colored central atom to make a model for all of these molecules. This is because the central atom in all of these molecules is the same, so it does not matter what color it is. The other atoms attached to the central atom will determine the shape of the molecule.
All the molecules have the same central atom because they are all hydrocarbons with the general formula CnH2n+2. The only difference between them is the number of carbon atoms that are present.For example, methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10) are all hydrocarbons, and they all have Carbon as their central atom. The number of hydrogen atoms in each molecule varies based on the number of carbon atoms present.For instance, Methane (CH4) has one carbon atom and four hydrogen atoms, Ethane (C2H6) has two carbon atoms and six hydrogen atoms, Propane (C3H8) has three carbon atoms and eight hydrogen atoms, and Butane (C4H10) has four carbon atoms and ten hydrogen atoms. Therefore, you can use the same central atom, Carbon (C), to create a model for all of these molecules.
For more such questions on Central atom
https://brainly.com/question/15088200
#SPJ11
what are two other things you could do to drive your reaction forward? these must be specific procedural steps you could do in the experimen
Answers
In an experiment, there are several things that you could do to drive the reaction forward. Two specific procedural steps that you could do in the experiment to drive the reaction forward are as follows:1. Increasing the temperature: The rate of the reaction increases with an increase in temperature.
The higher the temperature, the faster the particles will move, resulting in more collisions that are energetic enough to cause a reaction. If the temperature is lowered, then the reaction rate will slow down.2. Increasing the concentration of reactants: The rate of the reaction increases with an increase in the concentration of reactants.
If the concentration of reactants is high, then there will be more collisions between the molecules, resulting in a faster reaction. However, if the concentration is low, then there will be fewer collisions, resulting in a slower reaction. Thus, these are two specific procedural steps that you could do in the experiment to drive the reaction forward.
To know more about Reaction forward refer here:
https://brainly.com/question/8592296#
#SPJ11
what term refers to the ability of open systems to fight off deterioration, sustain themselves and grow? a. requisite variety b. network properties c. negative entropy d. modeling techniques
Answers
The ability of open systems to fight off deterioration, sustain themselves and grow is Negative Entropy. Correct answer is option C
Negative Entropy is an important concept in thermodynamics and physics, where it is defined as a decrease in the entropy of a system. Entropy is the measure of randomness or disorder in a system, so negative entropy indicates that a system is becoming more organized, or that it is moving away from equilibrium.
This can be seen in the evolution of life, where species become more complex and adaptive over time, as well as in the growth of technology, where innovations allow us to become more efficient and productive. In essence, Negative Entropy is the power that allows open systems to improve and evolve. Therefore Correct answer is option C
Know more about thermodynamics here:
https://brainly.com/question/1368306
#SPJ11
1) It takes 55.0 J to raise the temperature of an 11.0 g piece of unknown metal from 13.0∘C to 24.1 ∘C
What is the specific heat for the metal?
2) The molar heat capacity of silver is 25.35 J/mol⋅∘C
. How much energy would it take to raise the temperature of 11.0 g
of silver by 18.1 ∘C?
3) What is the specific heat of the silver?
Answers
The specific heat capacity of the metal is 4.98 J/(kg⋅K). It would take 46.7 J of energy to raise the temperature of 11.0 g of silver by 18.1 °C.
What is specific heat capacity?Specific heat capacity is the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin). It is a property of the substance and is usually denoted by the symbol c. The unit of specific heat capacity is J/(kg·K) or J/(kg·°C).
1. The specific heat of the metal can be calculated using the formula:
q = mcΔT
In this case, q = 55.0 J, m = 11.0 g = 0.0110 kg, ΔT = (24.1 - 13.0) = 11.1 °C = 11.1 K.
Substituting these values into the formula, we get:
55.0 J = (0.0110 kg) c (11.1 K)
Solving for c, we get:
c = 4.98 J/(kg⋅K)
Therefore, the specific heat of the metal is 4.98 J/(kg⋅K).
2. First, we need to convert the mass of silver from grams to moles:
n = m/M
where n is the number of moles, m is the mass in grams, and M is the molar mass of silver. The molar mass of silver is 107.87 g/mol, so we have:
n = (11.0 g)/(107.87 g/mol) = 0.102 mol
Substituting the values of m and ΔT, we get:
q = (0.102 mol)(25.35 J/mol⋅∘C)(18.1 ∘C) = 46.7 J
Therefore, it would take 46.7 J of energy to raise the temperature of 11.0 g of silver by 18.1 ∘C.
3. The specific heat of silver can be calculated using the formula:
c = C/M
where c is the specific heat, C is the molar heat capacity, and M is the molar mass.
The molar heat capacity of silver is given as 25.35 J/mol⋅∘C, and the molar mass of silver is 107.87 g/mol.
Converting the molar mass to kilograms per mole, we get:
M = 107.87 g/mol = 0.10787 kg/mol
Substituting the values of C and M, we get:
c = (25.35 J/mol⋅∘C)/(0.10787 kg/mol) = 234.9 J/(kg⋅K)
Therefore, the specific heat of silver is 234.9 J/(kg⋅K).
To find out more about specific heat capacity, visit:
https://brainly.com/question/29766819
#SPJ1
How many moles are there in 6.02 x1023 molecules of oxygen?
Answers
Answer: 1 mole
Explanation:
Avogadros Number; 6.02x 10^23 molecules in 1 mole
you have a stock solution of 0.6 molar sucrose, and want to prepare 3 ml of 0.24 molar sucrose solution. what are the correct amounts of 0.6 m sucrose and water that you will need to use?
Answers
Answer : To prepare 3 mL of 0.24 M sucrose solution from a stock solution of 0.6 M sucrose, 1.2 mL of the stock solution and 1.8 mL of water should be used.
The amount of 0.6 Molar sucrose needed to prepare 3 mL of 0.24 Molar sucrose solution, as well as the volume of water required, can be calculated using the M1V1 = M2V2 formula. Where M1 is the molarity of the stock solution, V1 is the volume of the stock solution required, M2 is the desired molarity of the solution to be prepared, and V2 is the volume of the solution to be prepared.
Given that the stock solution of sucrose is 0.6 M, and we need to prepare 3 mL of a 0.24 M solution, we can use the formula:
0.6 M x V1 = 0.24 M x 3 mL Solving for V1:
V1 = (0.24 M x 3 mL)/0.6 M
V1 = 1.2 mL
This means that 1.2 mL of the stock solution of 0.6 M sucrose is required to prepare 3 mL of 0.24 M sucrose solution.
The volume of water required can be calculated by subtracting the volume of the stock solution from the total volume of the solution to be prepared: Volume of water = 3 mL - 1.2 mL and Volume of water = 1.8 mL
Know more about sucrose solution here:
https://brainly.com/question/3850162
#SPJ11
Compared to the velocity of an earthquake’s S-wave, the velocity of the P-wave in the same material is
Answers
Answer:
usually faster. The P-wave is a compressional wave, meaning it is a wave of compression and expansion that travels through the material. It is also known as a primary wave, and it is the fastest type of seismic wave. The S-wave, or secondary wave, is a shear wave, which is a wave that causes the material to oscillate perpendicular to the direction of the wave. The S-wave is usually slower than the P-wave.
To neutralize the acid in 10.0 mL of 18.0 M H2SO4 that was accidentally spilled on a laboratory bench top, solid sodium bicarbonate was used. The container of sodium
bicarbonate was known to weigh 155.0 g before this use and out of curiosity its mass was measured as 144.5 g afterwards. The reaction that neutralizes sulfuric acid this way is as follows: H2SO4 + 2 NaHCO3 --> Na2SO4 + 2 CO2 + 2 H2O
Was sufficient sodium bicarbonate used? Calculate the limiting reactant and the maximum yield in grams of sodium sulphate.
Answers
8.88 g is the greatest yield of Na2SO4 that may be produced. As a result of using less NaHCO3 than is required to fully react with the H2SO4, the actual number of NaHCO3 used.
Why is bicarbonate important to the body?The body requires the base chemical bicarbonate to maintain a healthy acid-base balance. Your body's natural pH balance keeps it from becoming overly acidic, which can lead to a variety of health issues. By eliminating extra acid, the kidneys and lungs maintain a normal blood pH.
What occurs when the bicarbonate level is low?Metabolic acidosis is indicated by low blood bicarbonate levels. It is an alkali, the antithesis of acid, and it can counteract acid. Our blood's acidity is kept under control by it.
To know more about Bicarbonate visit:
https://brainly.com/question/8560563
#SPJ1
9. a 50 ml sample of an aqueous solution contains 1.08 g of human serum albumin, a blood-plasma protein. the solution has an osmotic pressure of 5.85 mmhg at 298 k. what is the molar mass of the albumin?
Answers
The molar mass of the albumin can be calculated by dividing the number of moles (1.08 g) by the molarity (0.0216 mol/L), which yields a molar mass of 49.54 g/mol.
The molar mass of the albumin can be calculated using the given data. First, calculate the molarity of the solution. Molarity = Number of moles/Volume of solution = 1.08 g/50 mL = 0.0216 mol/L.
The osmotic pressure of the solution can be calculated using the Van’t Hoff equation,
which states that osmotic pressure is equal to the molarity multiplied by the universal gas constant (R) multiplied by the temperature (T).
Therefore, osmotic pressure = 0.0216 mol/L × 8.3145 L.atm/mol.K × 298 K = 5.85 mmHg.
The molar mass of the albumin, rearrange the osmotic pressure equation to solve for molarity, molarity = osmotic pressure/RT = 5.85 mmHg/(8.3145 L.atm/mol.K × 298 K) = 0.0216 mol/L.
The molar mass of the albumin can be calculated by dividing the number of moles (1.08 g) by the molarity (0.0216 mol/L), which yields a molar mass of 49.54 g/mol.
The molar mass of the albumin can be calculated by first calculating the molarity of the solution, which is equal to the number of moles divided by the volume of the solution.
The osmotic pressure of the solution can then be calculated using the Van't Hoff equation, which states that osmotic pressure is equal to the molarity multiplied by the universal gas constant and the temperature.
The molar mass of the albumin can then be calculated by rearranging the osmotic pressure equation to solve for molarity and then dividing the number of moles by the molarity. This yields a molar mass of 49.54 g/mol.
to know more about albumin refer here:
https://brainly.com/question/18882874#
#SPJ11
the temperature of a constant volume of gas at 1.00 atm is 25 oc. in order to increase the pressure to 2.00 atm, what temperature is needed?
Answers
Answer: 323 degrees Celsius :)
Explanation:
What test in which the glucose molecules attached to hemoglobin a1 is being measured?
Answers
The test in which the glucose molecules attached to hemoglobin A1 is being measured is known as Hemoglobin A1c (HbA1c) test.
The Hemoglobin A1c (HbA1c) test is a blood test that calculates the average blood sugar level over the previous two to three months. It is used to diagnose prediabetes and type 2 diabetes. It's also utilized to check whether diabetes is under control for those who already have it.
The Hemoglobin A1c test result is expressed as a percentage. A normal Hemoglobin A1c level for someone without diabetes is typically less than 5.7 percent. The suggested target Hemoglobin A1c level for someone with diabetes is usually less than 7%.
A high Hemoglobin A1c level indicates that a person has a higher risk of diabetes complications. In people with diabetes, high Hemoglobin A1c levels can increase the likelihood of heart disease, kidney disease, nerve damage, and vision issues.
Therefore the answer is Hemoglobin A1c (HbA1c) test.
To learn more about Hemoglobin A1c (HbA1c) test refer: https://brainly.com/question/27186330
#SPJ11
which of the following is the most likely range of values for human body density? a. 0.900 - 1.100 g/cc b. 1.09 - 1.105 g/cc c. 0.99 - 1.02 g/cc d. 1.02-1.08 g/cc
Answers
The most likely range of values for human body density is 0.900 - 1.100 g/cc.(A)
Option (b) 1.09 - 1.105 g/cc is not the most likely range of values for human body density.
Option (c) 0.99 - 1.02 g/cc and option (d) 1.02-1.08 g/cc are also not the most likely range of values for human body density.
Body density is the mass of the human body divided by the volume it occupies. The density of the human body depends on the mass and volume of the body's internal organs, muscle mass, and the amount of adipose tissue present in the body.
The density of the human body typically ranges from 0.900 g/cc to 1.100 g/cc. This range may vary depending on several factors, including age, gender, body composition, and other health factors.
However, the most likely range of values for human body density is 0.900 - 1.100 g/cc.
To know more about adipose tissue click on below link:
https://brainly.com/question/30782617#
#SPJ11
alcl3 or fecl3 are also commonly used as catalysts for friedel-crafts alkylations. why might we opt to start with al as the catalyst starting point instead?
Answers
AlCl₃ is preferred as a catalyst for Friedel-Crafts Alkylations because it is more stable than FeCl₃.
AlCl₃ is also much easier to handle than FeCl₃ and has a higher boiling point. Additionally, it is less likely to cause a side reaction than FeCl₃ and more likely to produce higher yields.
Therefore, AlCl₃ is the more preferred catalyst when performing Friedel-Crafts Alkylations.
AlCl₃ is a strong Lewis acid, meaning that it can easily accept electrons from other species in order to form a coordinate covalent bond. This allows it to act as a catalyst for Friedel-Crafts Alkylations by providing a Lewis acid environment in which the reaction can take place.
AlCl₃ is less reactive than FeCl₃, which means that it is less likely to cause a side reaction. Additionally, AlCl₃ is more stable than FeCl₃ and has a higher boiling point, making it easier to handle. AlCl₃ is also more likely to produce higher yields when performing Friedel-Crafts Alkylations, making it the preferred catalyst in this reaction.
To know more about Friedel-Crafts Alkylations click on below link:
https://brainly.com/question/30884625#
#SPJ11